Alcohol Fuel Cell Sensor
Alcohol Fuel Cell Sensor – Ultrasonic Fuel Cell Catalyst Coating Systems – Cheersonic
First invented by Sir William Grove in 1839, fuel cells have been used in many devices in recent years as gas sensors for detecting oxidizable gases or vapors. Essentially, each fuel cell gas sensor consists of a working electrode (or anode) and a counter electrode (or cathode) separated by an electrolyte, usually a porous solid, which is impregnated with an acidic electrolyte. Electrochemical oxidation of the fuel gas results in a potential difference that causes electrons to flow from the anode to the cathode, and this current and/or potential difference can be detected.
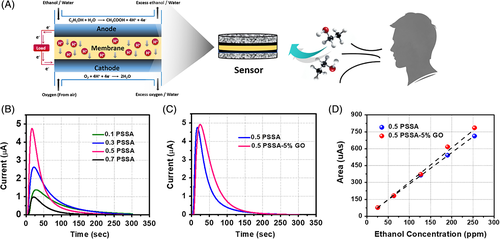
Taking the ethanol gas sensor as an example, an electrochemical gas sensor based on fuel cell technology is demonstrated together with a membrane for the detection of alcohol, showing excellent sensitivity, good linearity, and a detection limit of ethanol as low as 25 ppm.
The membrane electrode assembly (MEA) is the core component of the sensor consisting of a PVA-PSSA-GO membrane and two commercially available gas diffusion electrodes (GDE). The PVA-PSSA-GO membrane is sandwiched between two electrodes, and both the anode and cathode are coated with Pt/C catalyst. As shown, the sensor performance was investigated in a system consisting of the sensor along with an alcohol simulator, flow meter, moisture separator, data logger, and computer analyzer. Alcohol simulators are used to simulate the humidity/temperature of human breath. When a certain amount of air is pumped through the alcohol simulator, a certain concentration of ethanol vapor will be carried out with the air. As shown in the figure, as soon as ethanol vapor diffuses to the anode, an ethanol oxidation reaction (EOR) occurs immediately, producing protons and electrons. The protons transport across the PVA-PSSA-GO electrolyte membrane and react with oxygen at the cathode, triggering the oxygen reduction reaction (ORR) and producing water. The electrons transported from the external circuit are collected as an electrical signal, which is positively linear with the concentration of the input ethanol vapor. By analyzing the electrical signal in a computer analyzer, a typical response current curve is obtained and four different parameters are characterized: peak height, peak area, response time, and recovery time. Theoretically, there is a linear relationship between the ethanol concentration and the peak area corresponding to the amount of transferred electrons.
Ultrasonic spray fuel cell catalyst coating system can produce highly uniform, repeatable and durable coatings. Our ultrasonic spraying can well control coating properties, significantly reduce material usage, and reduce maintenance and downtime. Uniform catalyst coatings are deposited onto PEM fuel cells, GDLs, electrodes, various electrolyte membranes, and solid oxide fuel cells with suspensions containing carbon black inks, PTFE binder, ceramic slurries, platinum and other precious metals.
Cheersonic is the leading developer and manufacturer of ultrasonic coating systems for applying precise, thin film coatings to protect, strengthen or smooth surfaces on parts and components for the microelectronics/electronics, alternative energy, medical and industrial markets, including specialized glass applications in construction and automotive.
Chinese Website: Cheersonic Provides Professional Coating Solutions

